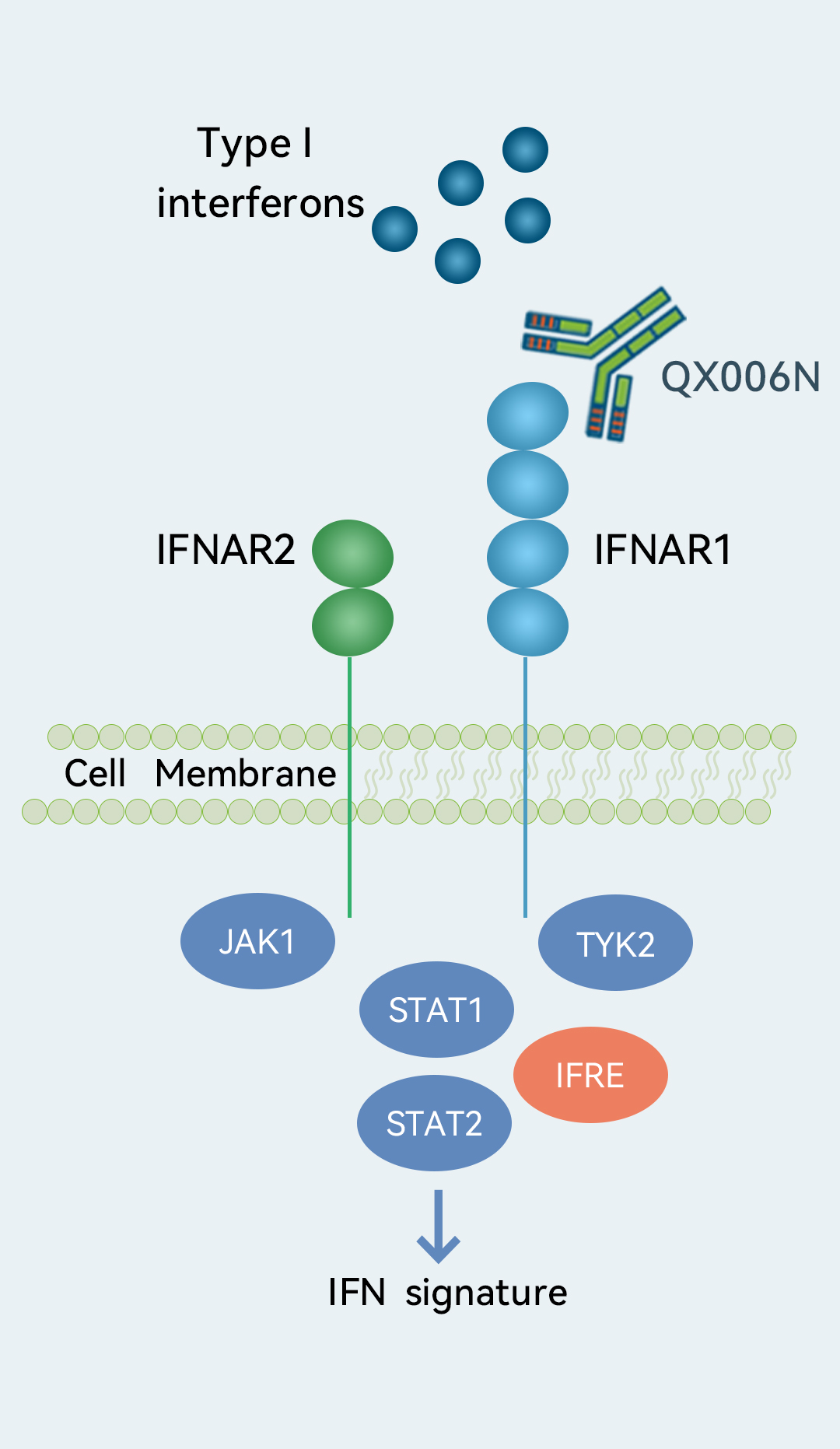
Target
Target- IFNAR1
 Generic Name
Generic Name- Recombinant humanized anti IFNAR1 monoclonal antibody injection
 Potential Indication
Potential Indication- SLE
We are developing QX006N, an IFNAR1-targeting mAb, for the treatment of SLE. SLE has been a difficult indication for new drug development. SAPHNELO (anifrolumab), a first-in-class IFNAR1 inhibitor, was approved by the FDA in 2021,making it the only new SLE treatment in more than 10 years. (The previous approved SLE drug, belimumab, was, at its time, the first approved SLE drug in 50 years.) Anifrolumab demonstrated clear clinical benefit in patients with moderate-to-severe SLE in a Phase III study (TULIP-2) and a Phase IIb study (MUSE). As of the Latest Practicable Date, there were no approved IFNAR1 inhibitors in China for the treatment of SLE, indicating a huge underserved market. As of the same date, our QX006N was one of the only two IFNAR1 inhibitors developed by Chinese domestic companies that had entered the clinical stage for SLE in China. QX006N showed a good safety profile based on preliminary results from our Phase Ia clinical trial, and promising potency and affinity comparable to those of an internally prepared anifrolumab analog in our preclinical studies. We expect to complete our ongoing Phase Ia clinical trial in healthy subjects in the second quarter of 2023. We also initiated a Phase Ib clinical trial in SLE patients in March 2023.
According to Frost & Sullivan, the prevalence of SLE in China is relatively stable, which was approximately 1 million in 2021 and is estimated to increase to 1.1 million in 2030. The SLE drug market in China was US$0.4 billion in 2021 and is estimated to reach US$3.4 billion in 2030, at a CAGR of 26.8%.