Target
Target- IL-12/IL-23p40
 Generic Name
Generic Name- ustekinumab
 Potential Indication
Potential Indication- moderate-to-severe plaque Ps UC/CD
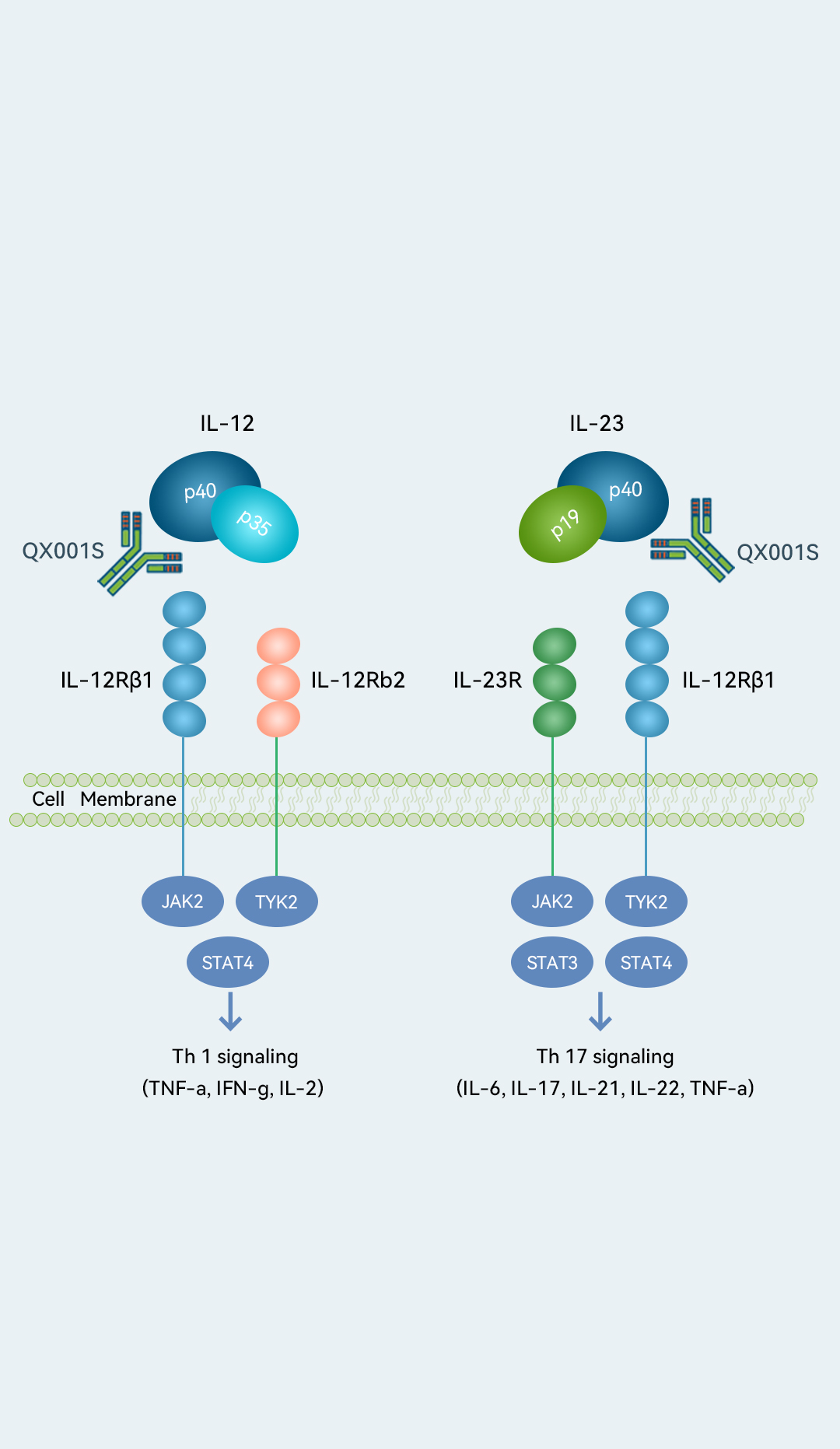
QX001S is our first expected commercial drug and potentially China’s first approved ustekinumab biosimilar.
Initially approved by the FDA in 2009, ustekinumab was the first biologic treatment to selectively inhibit the IL-23 and IL-12 pathways and has been widely regarded as one of the major treatments for psoriasis (Ps) worldwide. In 2021, it recorded sales of US$9.1 billion globally and ranked the ninth best-selling drug worldwide in the same year, according to Frost & Sullivan. In our preclinical studies and Phase I clinical trial for Ps, QX001S demonstrated a safety and PK profile comparable to that of ustekinumab. We are currently conducting a Phase III clinical trial of QX001S for Ps. In October 2022, this trial reached its primary endpoint as reviewed by the IDMC. We understand that Zhongmei Huadong, a subsidiary of Huadong Medicine and our commercialization partner for QX001S, plans to submit a BLA in China in the third quarter of 2023 and begin commercializing QX001S in the second half of 2024. We expect QX001S to be an affordable drug for a broad section of Ps patients. We also plan to develop QX001S for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD).
According to Frost & Sullivan, the prevalence of Ps in China was 6.7 million in 2021 and is anticipated to reach 6.9 million in 2030. The Ps drug market in China was US$1.1 billion in 2021, and is estimated to grow to US$9.5 billion in 2030, at a CAGR of 27.1%.There are two main types of biologic drugs approved for Ps in China, namely, TNF inhibitors and IL inhibitors. As TNF inhibitors have significant limitations, including multiple adverse effects and a high rate of non-responsiveness, IL inhibitors are considered second-generation biologic treatments for Ps that will become mainstream.